Virogin Announces Collaboration of Second-Generation VG201 with Akeso’s AK112 for Colorectal Cancer Liver Metastasis

April 16, 2025 Virogin Biotech, a clinical stage biotech company specializing in next-generation oncolytic viruses, today announced a landmark clinical collaboration with Akeso Inc. (9926.HK) to advance a multi‑center, open‑label Phase Ib/II study evaluating the combination of Virogin’s VG201 with Akeso’s breakthrough bispecific antibody Ivonescimab (AK112). This study targets advanced colorectal cancer liver metastasis (CRLM), […]
Virogin’s Oncolytic Virus VG161 Clinical Data Published in Nature: Ignites Hope for Advanced Liver Cancer Patients
On March 19, Nature published clinical research data by the First Affiliated Hospital of Zhejiang University on VG161, an oncolytic virus developed by Virogin Biotech. The study focused on patients with advanced hepatocellular carcinoma (HCC) who had failed multiple lines of treatment. Results demonstrated that VG161 not only exhibited excellent safety but also significantly extended […]
Breaking News | Virogin’s First Oncolytic Virus VG161 Receives Breakthrough Therapy Designation in China

Vancouver, BC Canada, September 6 2024 The first-in-class oncolytic virus developed by Virogin Biotech Co., Ltd. (“Virogin Biotech”), and exclusively licensed to CNBG-Virogin (Shanghai) Co., Ltd. (“CNBG-Virogin “) for clinical development and commercialization in the China region, was granted Breakthrough Therapy Designation (BTD) by the Center for Drug Evaluation (CDE) of the National Medical Products […]
Virogin Secures Oral Presentation at ESMO 2024 with New Clinical Data on VG201

Vancouver BC Canada, August 14, 2024 Virogin Biotech, a clinical-stage biotechnology company specializing in next-generation oncolytic viruses and mRNA technologies, proudly announces that its latest research on VG201, an advanced non-attenuated HSV-1 oncolytic virus product based on transcription-translation dual regulation (TTDR), has been selected for an oral presentation at the prestigious 2024 European Society for […]
Virogin Biotech Oncolytic Virus VG161 Benefit in HCC Patients Featured at ASCO 2024 and Case Reports in Sarcoma Published in Oncology Letters

Vancouver BC Canada, April 15, 2024 Virogin Biotech recently announced that their latest clinical trial results for advanced Hepatocellular Carcinoma (HCC) have been selected for poster presentation at the American Society of Clinical Oncology (ASCO) 2024. The trial features the innovative oncolytic virus VG161, which has demonstrated impressive survival benefit, in subgroup of patients with […]
Virogin Announces its Publication of HPV mRNA cancer vaccine expressing APC-targeting antigen in Immunology
Vancouver BC Canada, March 14, 2023 Virogin Biotech, a clinical-stage immuno-oncology company, announced its publication in a top peer-reviewed journal Immunology with the title “Prevention and treatment of HPV-related cancer through a mRNA vaccine expressing APC-targeting antigen”. This article highlights Virogin’s advancements in developing a new-generation therapeutic mRNA vaccine for HPV-related tumors. Our vaccine encodes […]
Virogin Announces its Publication of Homologous Prime Boost Strategy using HER-2 expressing Oncolytic HSV-1 in Vaccines
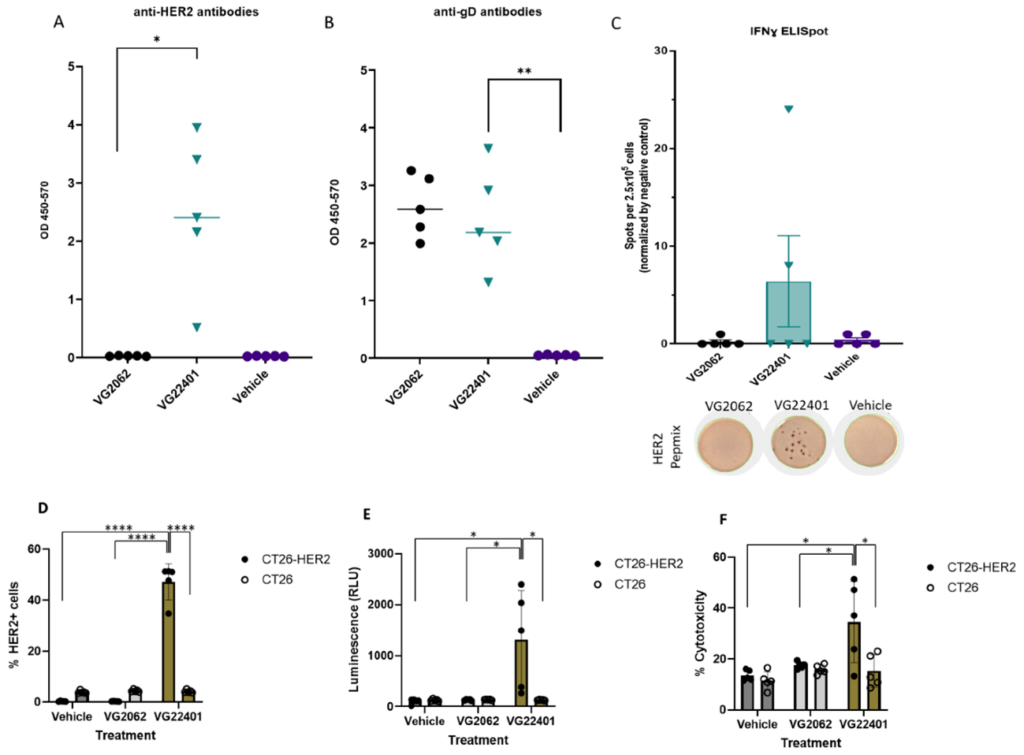
Vancouver BC Canada, December 05, 2023 Virogin Biotech, a clinical-stage immuno-oncology company, announced its publication in a top peer-reviewed journal Vaccines 2023, 11(12), 1805 with the title “Prophylactic Vaccination and Intratumoral Boost with HER2-Expressing Oncolytic Herpes Simplex Virus Induces Robust and Persistent Immune Response against HER2-Positive Tumor Cells”. This article focuses on the latest research […]
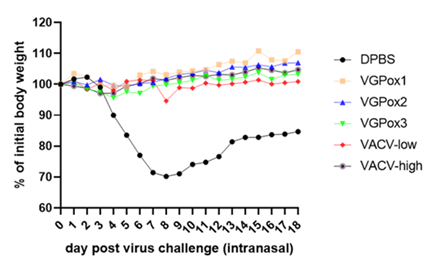
Virogin announced its publication of Monkeypox mRNA vaccine on Nature Communications
VIROGIN Receives FDA Fast Track Designation for VG161, a Novel Therapy Candidate for the Treatment of Advanced Hepatocellular Carcinoma
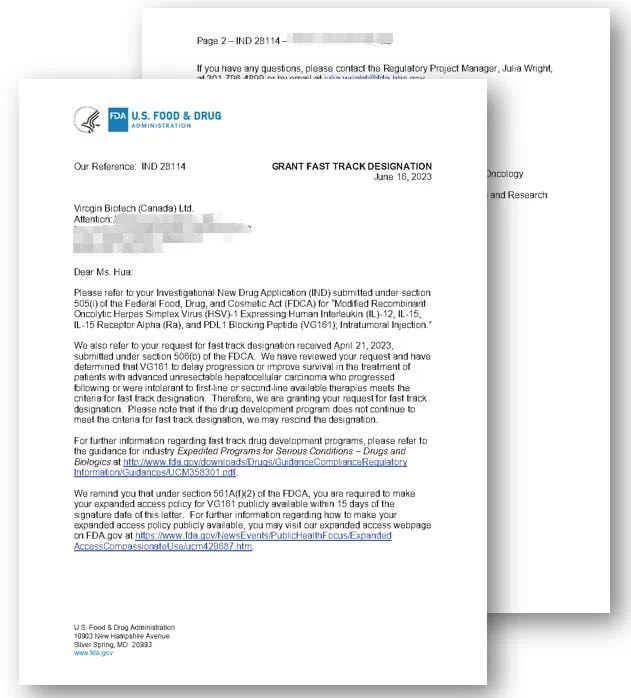
Vancouver, British Columbia, July 13, 2023 Virogin Biotech announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track designation for the investigation of VG161 for patients with advanced unresectable Hepatocellular Carcinoma. Hepatocellular carcinoma (HCC) is the most common type of primary liver cancers and accounts for 75–90% of all primary liver cancers, […]
VG161 granted for FDA Orphan Drug Designation

Vancouver, British Columbia, February 12, 2023 Virogin Biotech announced that it has received a formal letter response from the US Food and Drug Administration (FDA) Office of Orphan Products Development, of which its Class I innovative oncolytic virus product, VG161, has been granted for treatment of Intrahepatic Cholangiocarcinoma (ICC). Intrahepatic Cholangiocarcinoma (ICC) is a rare […]

