VG161, Virogin’s pioneering oncolytic virus built on the company’s proprietary SynerlyticTM Platform, is an attenuated herpes simplex virus type 1 (HSV-1) armed with multiple payloads of IL12 & IL15/IL15Ra and a unique PD-L1 blocking peptide. The neurovirulence of wild-type HSV-1 is mitigated by the deletion of ICP 34.5 gene. These payloads can synergistically stimulate both innate and adaptive anti-tumor immunity in the tumor microenvironment. VG161 has been confirmed to be safe and effective in many tumor xenograft mouse models and in GLP toxicity studies preclinically.
VG161 is being investigated as image guided, intratumoral injections (IT) in multiple Phase I & II clinical trials in US, Australia & China. Currently, there are 10 ongoing clinical trials (mono & combination therapy) across multiple geographies, with 2 of them conducted in Developed Markets.
VG161 has successfully completed a Phase I clinical trial in Australia and has entered a Phase 2 trial in the US. Our Phase 2 trial evaluates VG161 both as a mono and combination therapy at Mayo Clinic, with a focus on target indications Hepatocellular Carcinoma (HCC) & Intrahepatic Cholangiocarcinoma (ICC).

(Hepatocellular Carcinoma)
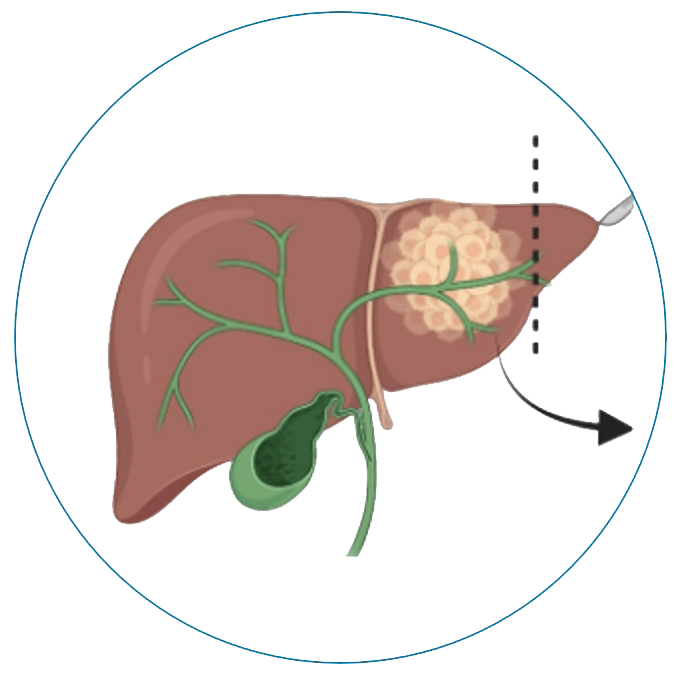
(Intrahepatic cholangiocarcinoma)



Our oncolytic HSV-1 VG161 not only does direct tumor directed killing (oncolysis) & but also activates innate & adaptive immune systems to boost the anti-tumor response. Hence, we believe IT (Intratumoral administration) of oncolytic virus has the potential to improve IO effectiveness & overcome resistance in earlier line settings.

In the response letter for Fast Track Designation for VG161, the FDA determined that VG161’s effect of PFS and OS in patients with advanced HCC (who progressed following or were intolerant to 1L & 2L available therapies) met the agency’s criteria for fast-track designation
Based on the single-agent antitumor activity demonstrated in hepatocellular carcinoma (HCC) in the latest line settings and the FDA’s Fast Track Designation, we believe that VG161 is well-positioned to address the high unmet medical need in HCC. As part of our Phase 2 plan in the US, we aim to recruit and dose a monotherapy safety run-in cohort for HCC and ICC at the Mayo Clinic before the year’s end. Additionally, we plan to initiate recruitment for the combination cohort of VG161 plus Nivolumab early next year.