Vancouver, British Columbia, July 13, 2023
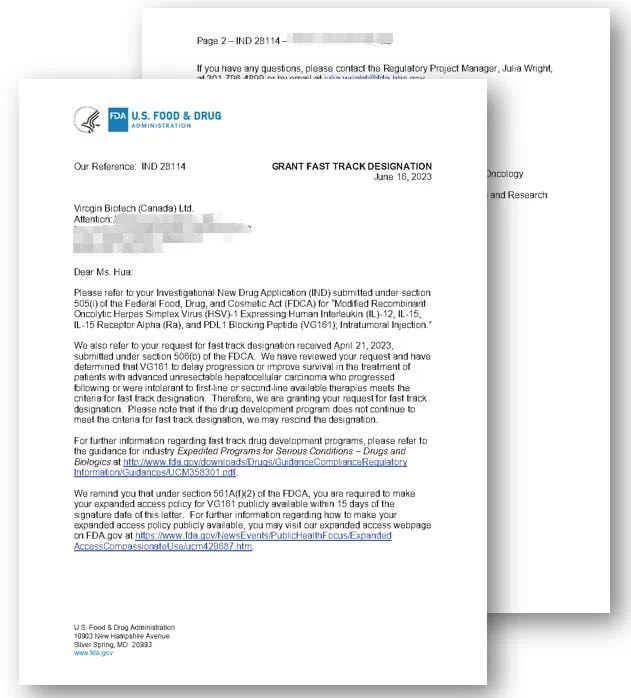
Virogin Biotech announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track designation for the investigation of VG161 for patients with advanced unresectable Hepatocellular Carcinoma.
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancers and accounts for 75–90% of all primary liver cancers, making it the third leading cause of cancer mortality worldwide. The incidence of HCC is increasing in the United States. In 2022, it was estimated that over 40,000 people in the United States would be diagnosed with liver cancer and despite all current therapies approximately 30,000 deaths were expected. Current therapies provide limited benefits to patients with lower survival rates for those who progress after available therapies and there is an urgent unmet medical need for developing new treatments for liver cancers.
In the response letter, the FDA determined that VG161’s effect of progression free survival and overall survival in patients with advanced HCC who progressed following or were intolerant to first- and second-line available therapies, met the agency’s criteria for fast-track designation.
“We are extremely encouraged by the FDA’s decision to grant Fast Track designation for VG161, and we look forward to expedite the clinical development for this product both in the US and globally” said Shah Rahimian, MD Virogin’s Chief Medical Officer of North America.
This designation approval was based on the preliminary data from VG161 clinical trials results carried out in China and Australia. A phase 2 study with VG161 is currently active at clinical sites in the US.
As a product of Virogin’s Synerlytic™ technology platform, VG161 has successfully completed several Phase I clinical trials, and entered Phase II clinical trials globally in 2022. In the same year, VG161 was approved by the Center for Drug Evaluation (CDE) in China for Phase I/II combination trials with other immunotherapy agents. Orphan Drug Designation was granted to VG161 for treatment of Intrahepatic Cholangiocarcinoma in February of 2023.
This designation empowers Virogin to explore more opportunities for communication and interaction with the FDA in future drug development and review process. Additionally, it allows for rolling submission of new drug research data to the FDA during the New Drug Application (NDA) or Biologics License Application (BLA) submission. These policies contribute to expediting the subsequent development and approval of VG161.
Virogin will further explore the clinical efficacy of VG161, establishing a solid foundation for clinical research that is dedicated to subsequent series of oncolytic virus products.
While steadily advancing its global clinical strategy, Virogin’s innovative research capabilities have been demonstrated with its robust preclinical-to-clinical translation and promising clinical research results.
About Virogin Biotech:
Virogin is developing two different platforms that are highly complementary: an oncolytic HSV-1 platform armed with multiple therapeutic payloads and genomic regulation to increase oncolytic effect and modulate the tumor microenvironment; and a discovery-stage mRNA tumor vaccine platform consisting of tumor vaccines to be used in combination with oncolytic viruses to augment anti-tumor activity.