-
- M22H04, the vaccine features an innovative antigen self-adjuvant design that effectively targets and activates antigen-presenting cells, leading to a heightened activation of anti-tumor immunity.
-
- Our therapeutic vaccine efficiently activates anti-tumor immune response, leading to regression of HPV-positive tumors, thus offering potential treatment for HPV-induced tumors.
- It effectively prevents the formation of HPV-positive tumors and reduces the risk of tumor recurrence, providing significant clinical implications for preventing the progression of precancerous lesions to tumors and preventing tumor recurrence after standard treatments.
Vancouver BC Canada, March 14, 2023
Virogin Biotech, a clinical-stage immuno-oncology company, announced its publication in a top peer-reviewed journal Immunology with the title “Prevention and treatment of HPV-related cancer through a mRNA vaccine expressing APC-targeting antigen”.
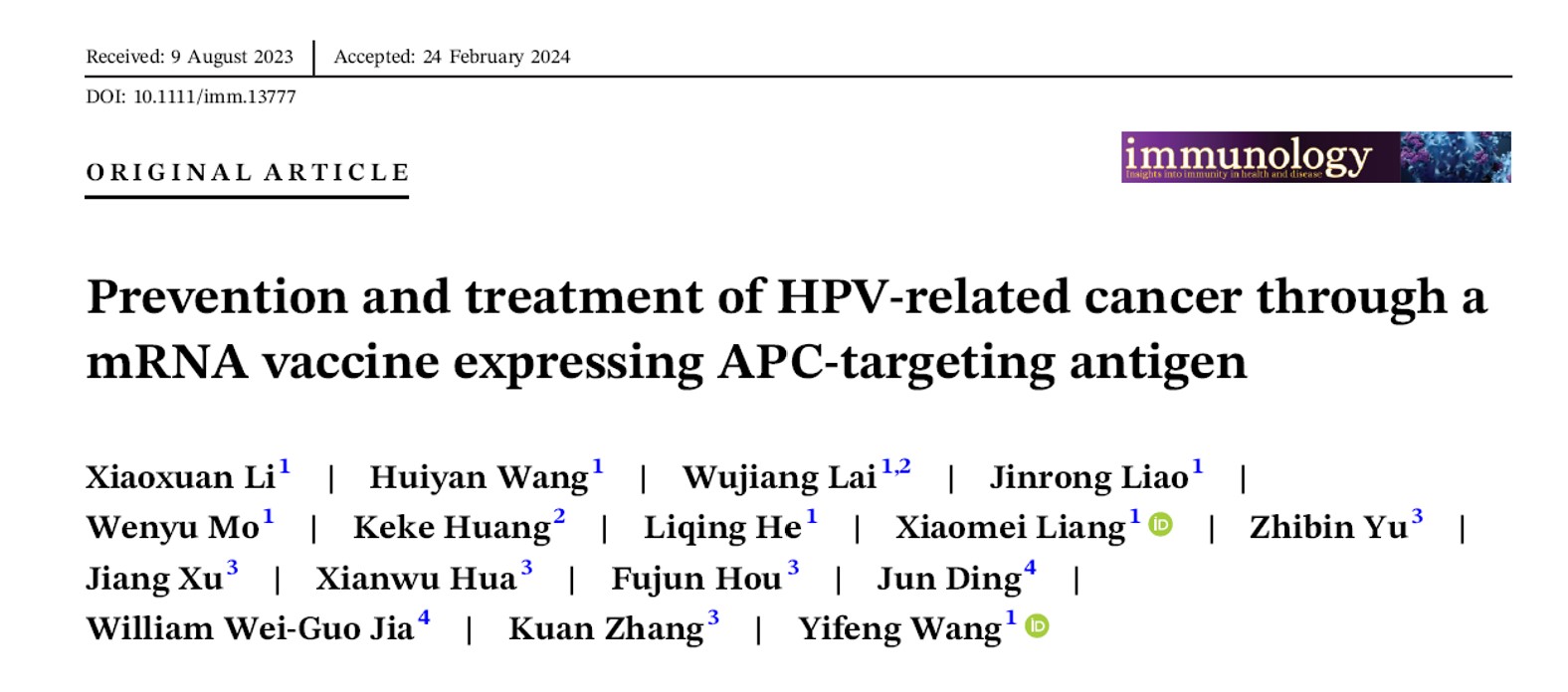
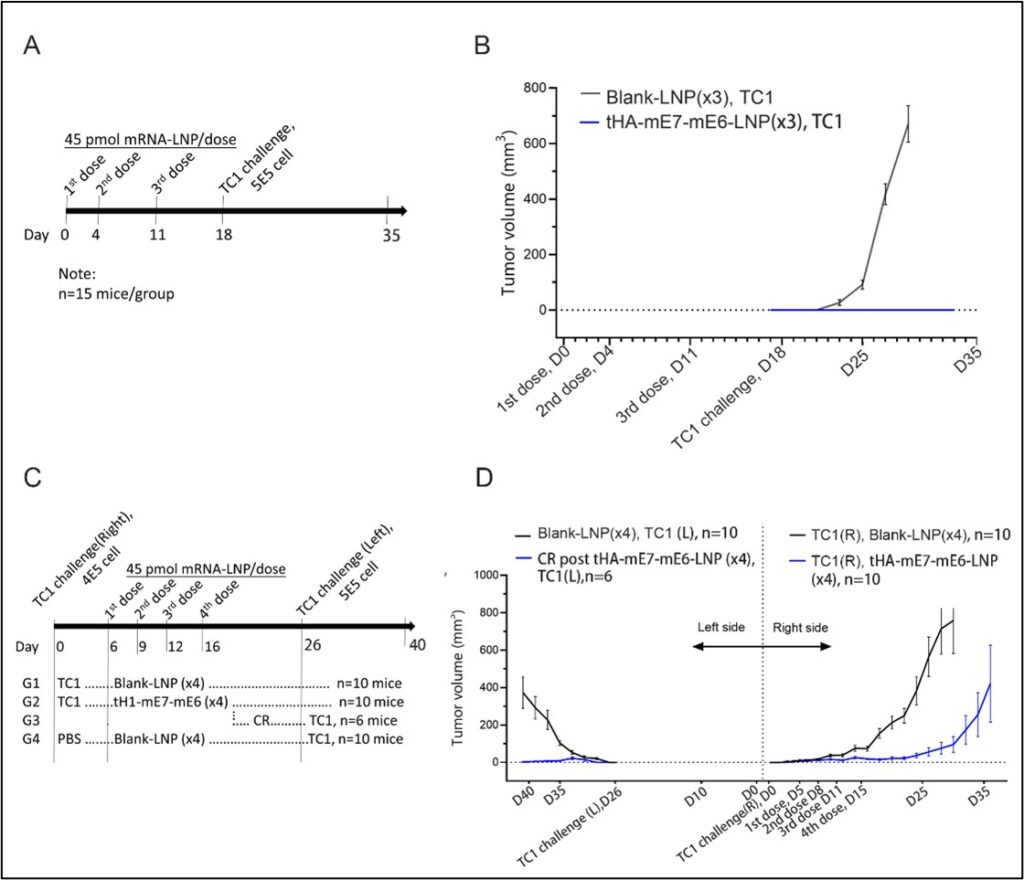
This article highlights Virogin’s advancements in developing a new-generation therapeutic mRNA vaccine for HPV-related tumors. Our vaccine encodes two modified oncoproteins, E6 and E7, fused with an antigen-presenting cell (APC)-targeting immunogen within the LNP-encapsulated mRNA vaccine. Our APC targeting antigen binds to the receptor on Dendritic Cells, enabling more efficient antigen presentation which potentially leads to efficient T cell stimulation. This approach enhances our vaccine’s therapeutic efficacy for both preventing and treating HPV-induced tumors.
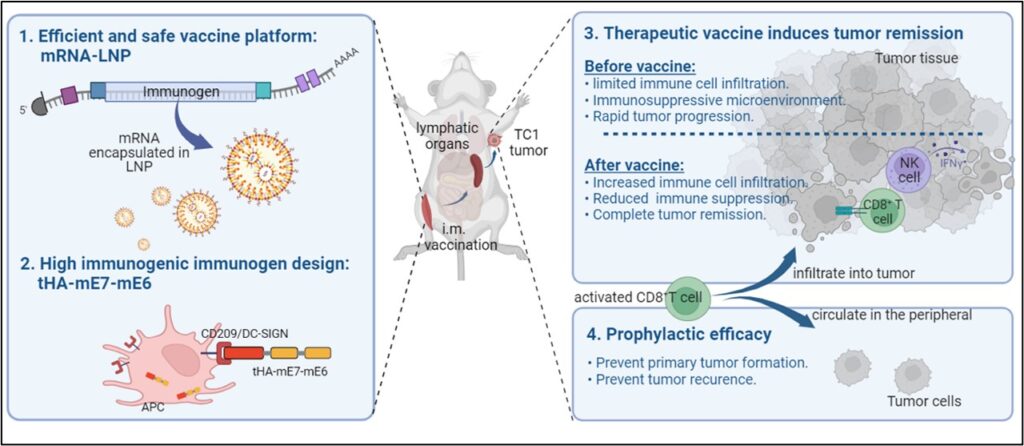
The study showcases the benefits of mRNA-LNP technology in cancer vaccine development, effectively stimulating anti-tumor immune responses more than traditional vaccine platforms like DNA and protein-based vaccines. In conclusion, our research demonstrates that our therapeutic mRNA cancer vaccine demonstrated a strong antigen-specific cytotoxic T-cell response and improved immune cell infiltration in the tumor, resulting in prevention and regression of E6 and E7+ tumors. The therapeutic effect on relapsing E6 and E7+ tumors was limited in tumors with high level of immune suppression, underscoring the importance of initiating the therapeutic vaccination at early cancer stages.
Figure 1. Overall Design of Virogin’s therapeutic vaccine (M22H04)
Background on HPV-induced tumors and development of therapeutic vaccines:
Human papillomavirus (HPV) is the most prevalent sexually transmitted viral infection globally and persistent HPV infection with high-risk HPV strains is a major cause of various cancers, including those of the head and neck, cervix, vagina, penis, and anus. Cervical cancer, driven by HPV in over 95% of cases, significantly threatens women’s health worldwide with a high mortality rate estimated at 342,000 annual deaths worldwide. Persistent HPV infection can lead to DNA alterations and abnormal cell proliferation, ultimately resulting in tumor formation. Although current HPV prophylactic vaccines effectively reduce infection rates, they do not protect individuals who already infected with the virus. This underscores the importance of developing therapeutic HPV vaccines to prevent tumor formation and treat HPV-related tumors effectively.
Currently, no therapeutic vaccines for HPV-related cancers or precancerous lesions are approved, highlighting the need for additional therapies to meet this high unmet need. Several companies worldwide are undergoing clinical trials, including Inovio and ApolloBio (developing DNA therapeutic vaccines requiring electroporation delivery), Vaccitech (with a viral-vector-based vaccine), Advaxis (with a bacterial vector-based vaccine), and BioNTech (developing mRNA-based vaccines in combination treatment with PD-1), are striving to fill this gap.
Virogin’s self-adjuvant mRNA-LNP therapeutic vaccine (M22H04) has shown promise in preventing and treating HPV-positive tumors. Through targeted binding and activation of antigen-presenting cells, our vaccine achieves efficient activation of anti-tumor immunity, leading to complete tumor regression and prevented tumor formation. This vaccine’s immunotherapeutic and preventive efficacy surpasses that of non-self-adjuvant mRNA-LNP vaccines. Additionally, it favorably affects the tumor microenvironment and promotes immune cell infiltration into tumors, as demonstrated in various animal models, including those for tumor prevention, treatment, and post-treatment tumor recurrence.
In conclusion, Virogin’s groundbreaking research in mRNA therapeutic vaccines for HPV-driven cancers underscores its capability to develop potent vaccines and highlights how its deep understanding of immunology contributes to pioneering vaccine approaches. The publication further solidifies Virogin’s contribution to the scientific community and potential impact on global health.
Figure 2. Comparison efficacy study of mRNA-LNP vaccine with and without APC-targeting domain.
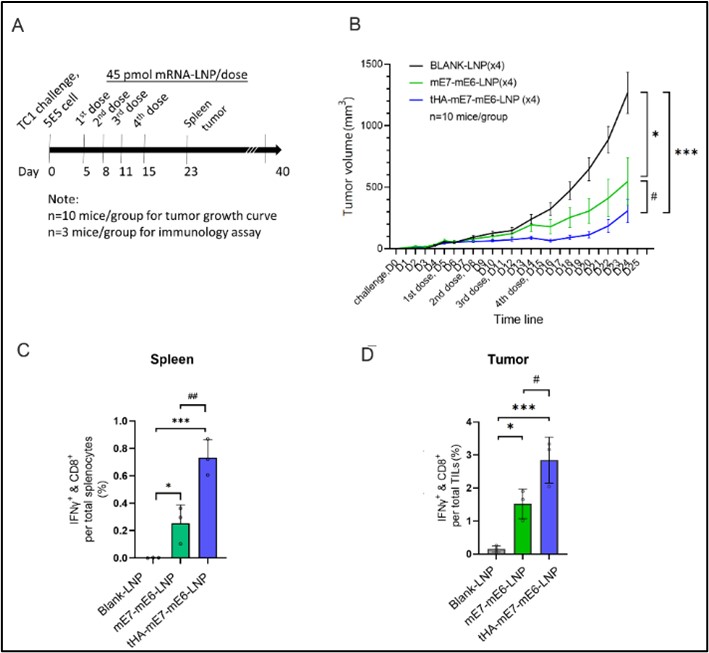
Figure 3. Correlative study of tumor immuno suppression and mRNA vaccine therapeutic efficacy.
Figure 4. Prophylatic efficacy study of Virogin’s therapeutic vaccine.
About Virogin Biotech:
Virogin Biotech is a clinical-stage immuno-oncology company, with a primary focus on innovative HSV-1 based oncolytic virus and mRNA-based (including self-amplifying RNA/saRNA) therapeutics to generate robust and durable anti-tumor immune response against solid tumors. Both platforms exhibit a high degree of complementarity via a mechanism of heterologous prime boost. Yet each platform has ‘plug-to-play’ features to independently develop novel therapies against cancer and other indications. Our oncolytic HSV-1 platform has three candidates in clinical trials, with our most advanced asset in Phase 2. Candidates from our mRNA platform have also completed preclinical proof of concept.