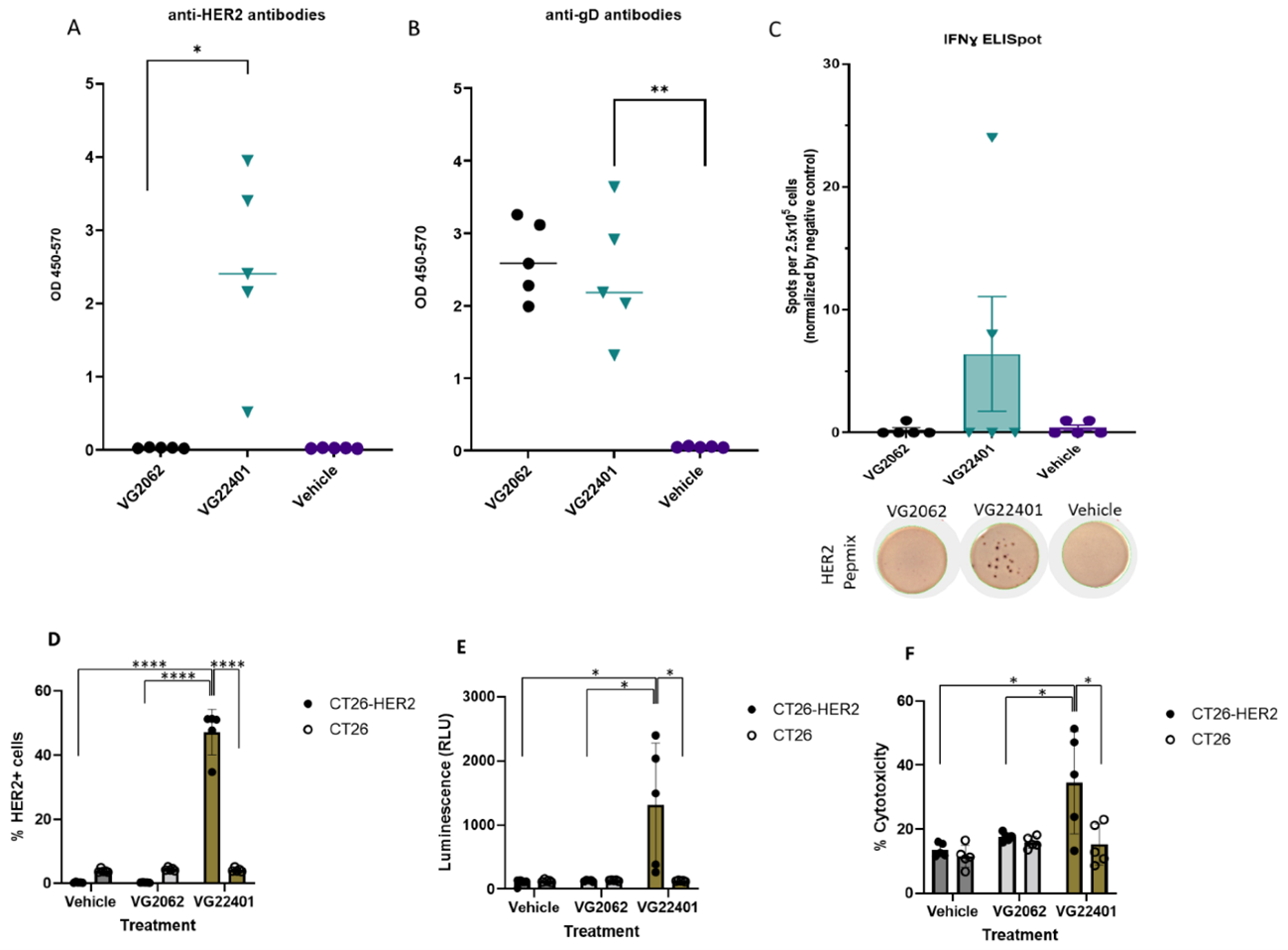
- Combining pre-immunization by HER2-expressing oHSV-1 with intratumoral oHSV-1 therapy not only triggers a robust humoral and cellular response against HER2(+), but also induces tumor antigen spread to initiate systemic antitumor immune response via oncolytic virus (OV)-mediated tumor cell lysis.
- After priming with a peripherally delivered tumor vaccine, intratumorally injected oncolytic viruses over-expressing a tumor-associated antigen along with the immunomodulatory cytokines, in this case IL12 and IL15RA1, can significantly boost OV-mediated antitumor immunity and efficacy.
- A novel oHSV-1 virus (VG401) expressing the human epidermal growth factor receptor 2 (HER2) exhibited efficient replication and HER2 payload expression.
Vancouver BC Canada, December 05, 2023
Virogin Biotech, a clinical-stage immuno-oncology company, announced its publication in a top peer-reviewed journal Vaccines 2023, 11(12), 1805 with the title “Prophylactic Vaccination and Intratumoral Boost with HER2-Expressing Oncolytic Herpes Simplex Virus Induces Robust and Persistent Immune Response against HER2-Positive Tumor Cells”.
This article focuses on the latest research progress of the company’s newest generation of oncolytic virus product VG401 (product code “VG22401”) that demonstrates the efficacy of a homologous prime–boost HSV-1 vaccination strategy.
“Our findings again emphasize the advantages of using HSV-1 OVs as tumor vaccines. Comparing to non-oncolytic tumor vaccines, OVs expressing tumor antigen not only generate the immunity against the specific tumor antigen but also against other tumor antigens through the process of antigen spread due to tumor lysis. Furthermore, our report was the first study to demonstrate homologous prime boost strategy using our oncolytic HSV- 1” said Dr. William Jia, the co-founder and Chief Scientific Officer of Virogin Biotech.
Oncolytic virotherapy is a potent weapon in cancer therapy. Virogin developed the novel VG401 (VG22401) recombinant oncolytic virus which is based on the company’s proprietary TTDR HSV-1 viral backbone with the addition of a HER2 payload. Besides directly attacking and lysing the tumor, VG401 locally expresses HER2 along with IL12 and IL15RA1 in the tumor mass, resulting in a strong immune response against both HER2 and other antigens released from the tumor cells.
Here are the key takeaways from our published article:
- VG401 exhibited efficient replication and HER2 payload expression in tumor cells.
- Mice immunized with VG401 showed significant binding of serum anti-HER2 antibodies to HER2-expressing tumor cells, inducing ADCC (antibody-dependent cell-mediated cytotoxicity) and CDC (complement-dependent cytotoxicity).
- Mice primed with VG401 and intratumorally boosted with the same virus showed enhanced antitumor efficacy in a bilateral syngeneic HER2(+) tumor model, compared to HER2-null backbone virus. This effect was accompanied by the induction of anti-HER2 T cell responses.
Intratumorally injected oncolytic viruses over-expressing a tumor-associated antigen can significantly boost OV-mediated antitumor immunity and efficacy after priming with a peripherally delivered tumor vaccine. This publication further solidifies our homologous prime-boost vaccination strategy for HSV-1, thus yielded unique and promising results for immuno-oncotherapy.
Figure 1: Induction of humoral and cellular immune response by an HSV-1 expressing HER2.
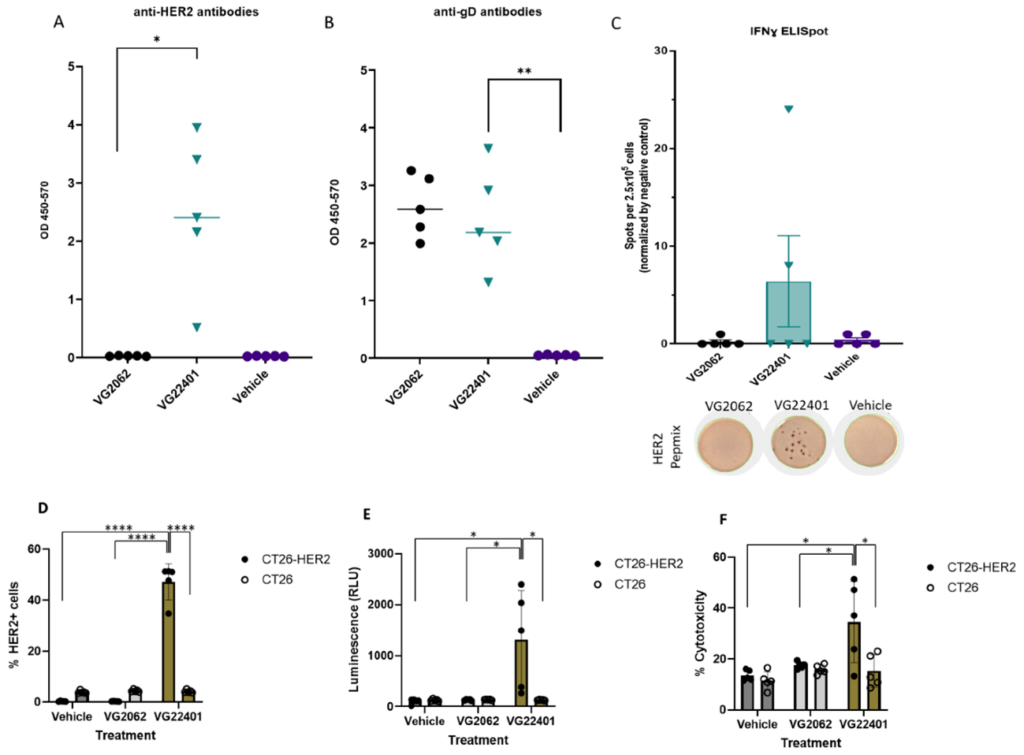
Figure 2: HER2 payload improves the antitumor effect of oHSV-1 and induces a T cell response in a HER2-vaccinated bilateral, syngeneic murine tumor model.
About Virogin Biotech:
Virogin Biotech is a clinical-stage immuno-oncology company, with a primary focus on innovative HSV-1 based oncolytic virus and mRNA-based (including self-amplifying RNA/saRNA) therapeutics to generate robust and durable anti-tumor immune response against solid tumors. Both the platforms exhibit a high degree of complementarity via a mechanism of heterologous prime boost. Yet each platform has ‘plug-to-play’ features to independently develop novel therapies against cancer & other indications. Our oncolytic HSV-1 platform has two candidates in clinical trials, with our most advanced asset in Phase 2. Candidates from our mRNA platform have also completed preclinical proof of concept.